 |
 |
|
|
 |
 |
EDITORIAL
|
SURVEY RESULTS – PART VIIIThe total sample of 118 consisted of 47 vagal afibbers, 23 adrenergic, 29 mixed, and 19 chronic. There were a total of 26 afibbers with blood type A, 28 with type O, 3 with type B, and 5 with type AB. Blood type A was most common among vagal afibbers (63%) while type O was most common among adrenergic (50%), mixed (58%) and chronic (71%).
Non-dietary correlations The correlation between the presence of amalgam (silver) dental fillings and length of time spent in fibrillation was confirmed in the larger sample. Afibbers without amalgams spent an average of 40 hours in fibrillation over the 6-month survey period while those with amalgam fillings spent 203 hours in afib. This difference was statistically significant (p=0.02). There was no indication that supplementing with magnesium or fish oil affected the severity of episodes. This is somewhat surprising as magnesium and fish oils have both been found effective in preventing other types of cardiac arrhythmias, especially ventricular arrhythmias. Perhaps is just goes to underscore that LAF truly is different from arrhythmias involving a diseased heart. Our findings, of course, do not detract from the other health benefits of magnesium and fish oil supplementation nor do they invalidate anecdotal evidence of their benefits. Nevertheless, magnesium and fish oils are clearly not "magic bullets" when it comes to preventing LAF episodes.
Effect of diet on episode frequency Afibbers with blood type A tended to have more episodes if they emphasized whole wheat products, fruits and vegetables, and in general, ate a high carbohydrate diet. Poultry consumption seemed to decrease the number of episodes. There were no obvious dietary triggers for blood type O.
Effect of diet on episode duration Diet did not seem to affect episode duration for blood type A, but afibbers with type O tended to have shorter episodes with an increased intake of omega-6 oils.
Effect of diet on time spent in fibrillation Diet did not seem to affect time spent in afib for blood type A, but afibbers with type O spent slightly longer in afib if they also had digestive problems.
Effect of diet on digestion There was no correlation between blood type and digestive problems.
Summary of diet survey It does seem though that pork, poultry, fish, vegetables, fruits and overall carbohydrate consumption could be important factors. It is conceivable that hormones in poultry could affect the autonomic nervous system, as could carbohydrates through the insulin spike caused by high glycemic index foods in particular. This, however, is pure speculation on my part and can only be confirmed or rejected via a much larger, better designed and professionally interpreted study.
To the best of my knowledge there have been no clinical trials or epidemiological studies to evaluate the use of pharmaceutical drugs or supplements in the prevention and cure of lone atrial fibrillation. Remarkable isn't it? Here is a debilitating disorder affecting thousands, if not millions, of people and no one - government laboratories, universities or pharmaceutical companies - has seen fit to try to come up with a solution! Granted there have been lots of studies concerning other cardiac arrhythmias and the use of antiarrhythmic drugs to treat them. Unfortunately, it is becoming increasingly clear that the results of these studies do not apply to the vast majority of lone afibbers whose hearts are free of underlying heart disease, but whose autonomic nervous systems are out of whack. Several supplements have been proposed as an aid in preventing LAF. The suggestions to use these supplements are generally based on the facts that they either support good health in general, support heart and circulatory system health or have been found useful in the treatment of other more serious cardiac arrhythmias such as ventricular tachycardias. It is important to keep this in mind as we discuss the various supplements. To the best of my knowledge none of the supplements discussed here have been specifically evaluated for use in the prevention of LAF.
A. Multivitamins
Vitamins
Essential Minerals
* preferably together with other carotenes such as lycopene, alpha-carotene, and
zeaxanthin In addition you need to make sure that your intake of the two major antioxidants, vitamins C and E, is adequate. Supplementation with the water-soluble vitamin C should be spread throughout the day (500 mg of ascorbic acid or calcium ascorbate with each meal is a common recommendation) or use timed-release capsules. Vitamin E can be taken just once a day (400- 800 IU per day of natural vitamin E [d-alpha-tocopherol or d-alpha-tocopherol acetate or succinate] is a common recommendation).
B. Vitamin C A recent study shows that people who supplement with more than 700 mg/day of vitamin C have a 62 per cent lower risk of dying from heart disease than do people with a daily intake of 60 mg/day or less[13]. Supplementation with 2 grams/day has been found to reduce adhesion of monocytes (white blood cells) to the lining of blood vessels and thereby reduce the risk of atherosclerosis[10,11,14]. Vitamin C supplementation (2 grams/day) also effectively reverses the vasomotor dysfunction often found in patients with atherosclerosis[15]. Research carried out in Japan has shown that restenosis (reclosing of opened arteries) after angioplasty can be significantly reduced by supplementing with ascorbic acid (500 mg/day)[16]. Some very recent research raises the tantalizing possibility that vitamin C (calcium ascorbate) may directly help control certain types of cardiac arrhythmias. Researchers at the Cleveland Clinic have found that patients undergoing bypass surgery have a 50% less risk of going into arrhythmias after the operation if they supplement with 2 grams/day of calcium ascorbate the day prior to surgery and for 5 days following surgery. The researchers conclude that oxidative stress is behind the post-surgery arrhythmias and that vitamin C may be helpful in preventing these arrhythmias[17]. A scientific advisory panel to the U.S. Government sponsored Alliance for Aging Research has recommended that all healthy adults increase their vitamin C intake to 250-1000 mg/day[18]. Although there has been some concern that people suffering from hemochromatosis (a tendency to iron overload) may be sensitive to high dosages of vitamin C most researchers now agree that vitamin C is entirely safe even in daily quantities of 10 grams or more[19-21]. Because vitamin C is water-soluble it should be taken throughout the day (500 mg of ascorbic acid or calcium ascorbate with each meal is a common recommendation). An alternative approach is to use a time-release vitamin C capsule[22].
C. Vitamin E Vitamin E is also highly effective in warding off a heart attack. Researchers at Cambridge University in England reported in 1996 that patients who had been diagnosed with coronary atherosclerosis could lower their risk of having a heart attack by 77 per cent by supplementing with 400 IU or 800 IU/day of natural source vitamin E[29]. Very recently researchers at the Toyama Medical University in Japan reported that patients with unstable angina could reduce their risk of angina attacks by a factor of six by supplementing with vitamin E (300 mg/day of alpha-tocopherol acetate)[30]. Supplementation has also been found useful in preventing complications after heart surgery and helps slow the restenosis (reblockage) of arteries subjected to angioplasty[26,27,31]. Italian researchers recently reported that older people with high blood levels of vitamin E have a 10 times lower risk of suffering a cardiovascular event than do people with lower levels[32]. It is clear that vitamin E is an extremely important factor in human health. Most studies involving vitamin E supplementation have used amounts between 100 IU/day and 800 IU/day and 400 IU/day is now considered to be a basic, safe and adequate dosage for an average, healthy person[33-37]. The optimum intake for an individual, however, depends on many factors including the intake of polyunsaturated fatty acids and the degree of exposure to air pollution and toxic chemicals. Higher dosages may be indicated for women suffering from premenstrual or menopausal problems, for smokers, for people engaging in heavy, outdoor exercise, and for people having a family history of cancer. Large, well-controlled studies of vitamin E supplementation have shown it to be non-toxic in intakes as high as 3200 IU/day[36,38]. However, most researchers caution against daily intakes higher than 800-1200 IU/day for extended periods[33,34,37]. It is also recommended that the progression to a daily dose of 400 IU be gradual as should any decrease in intake. Some very recent research has shown that vitamin E in dosages higher than 1600 IU/day may have a prooxidative effect. However, this effect can be avoided by always taking adequate amounts of vitamin C when supplementing with vitamin E[39,40]. There are some cases in which high dosages (more than 30 IU/day) are contraindicated. Medical advice concerning dosage should be sought by individuals having high blood pressure, those taking anticoagulant drugs (Coumadin, warfarin) or having a tendency to prolonged bleeding, those having a vitamin K deficiency, and those suffering from rheumatic heart disease, an overactive thyroid or diabetes[31,33,36]. Inorganic iron (ferrous sulphate) destroys vitamin E and birth control pills deactivate it to some degree. So it should be taken with the main meal (to optimize absorption) and at least six hours before or after taking an iron supplement or a birth control pill. Natural vitamin E comes in several forms; d-alpha-tocopherol (100 mg=149 IU), d-alpha- tocopherol acetate (100 mg=136 IU), and d-alpha-tocopherol succinate are the most common. The "d" designation in front indicates that the products are derived from natural sources such as vegetable oils or wheat germ. A prefix of "dl", such as dl-alpha-tocopherol, shows that the vitamin has been synthesized from a petroleum base. The synthetic form is far less effective than natural one. Recent research has also shown that expensive water-soluble forms are no more effective than the regular fat-soluble forms[31,41-44]. The benefits of an adequate vitamin E intake cannot be over-emphasized. Unfortunately, it is quite impossible to get enough from even the most well-balanced diet. To obtain a daily intake of 400 IU it would be necessary to consume 200 cups of brown rice, 10 cups of almonds, 80 cups of cooked spinach or 12 tablespoons of unrefined, fresh wheat germ oil every day. Supplementation is clearly necessary. A daily intake of 400 IU/day of natural vitamin E combined with 250-1000 mg/day of vitamin C will help protect you against heart disease, cancer, and many other degenerative diseases.
D. Coenzyme Q10 Heart attacks and strokes produce a burst of free radicals (ischemia-reperfusion), which can result in extensive tissue damage. Patients with high CoQ10 levels suffer less damage from these events and Japanese researchers have found that supplementation prior to and immediately following open-heart surgery is highly beneficial in preventing reperfusion injury - a common complication in heart surgery[45,50,51,54,55]. Supplementation with CoQ10 has also been found beneficial in patients with chronic stable angina, mitral valve prolapse and irregular heart beat (arrhythmias)[45,49-51,56-58]. Coenzyme Q10 has also proven useful in the treatment of various cardiomyopathies (diseases of the heart muscle that reduce its pumping capacity). Studies have shown that supplementation with as little as 100 mg/day for 12 months results in better pumping capacity (increased ejection fraction), increased muscle strength and improved breathing[45,49,50,59]. Several studies indicate that CoQ10 may be beneficial in the treatment of hypertension (high blood pressure). A study of 109 patients with long-standing, essential hypertension, who were on antihypertensive drugs, concluded that supplementation with an average of 225 mg/day improved functional status, allowed about half the patients to discontinue most of their blood pressure medications and resulted in an average decrease of systolic blood pressure from 159 to 147 mm Hg and a diastolic pressure decrease from 94 to 85 mm Hg. Smaller, more recent Japanese studies have confirmed these findings[45,49-51,60-62]. Studies at the University of Ancona in Italy have provided evidence that CoQ10 supplementation reduces blood levels of epinephrine (adrenalin) and other catecholamines; this is believed to be partly responsible for the drop in blood pressure and may also explain why CoQ10 is effective in reducing the incidence of certain types of arrhythmias[45,63]. The body can synthesize coenzyme Q10 and it is also found in several dietary sources, notably organ meats. The level of CoQ10 in human organs peaks around the age of 20 years and then declines fairly rapidly. The decrease in CoQ10 concentration in the heart is particularly significant with a 77-year-old person having 57 per cent less in the heart muscle than a 20-year-old[64]. Some experts involved in CoQ10 research believe that many people, especially older people and people engaging in vigorous exercise may be deficient and may benefit from supplementation. The recommended daily dosage for health maintenance is 30 mg; however, considerably higher amounts are required in the treatment of the various diseases for which supplementation has been found beneficial[45,49,65,66]. It should be taken with a meal containing some fat or even better, in combination with soy or vegetable oil which enhances its absorption quite substantially[49]. CoQ10 supplements are readily absorbed by the body and no toxic effects have been reported for daily dosages as high as 300 mg. The safety of CoQ10, however, has not been established in pregnancy and lactation, so caution is advised here until more data becomes available[49,51].
E. Alpha-lipoic acid Oxidative stress is intimately involved in the aging of the heart and results in significant DNA damage to heart cells. Researchers at the Linus Pauling Institute recently reported that supplementation with alpha-lipoic acid (in laboratory animals) reduces DNA damage and oxidative stress to the level observed in young animals[68]. German researchers have found that supplementation improves glucose utilization and cardiac autonomic function in diabetics[69-71]. Chinese researchers using animal models found that lipoic acid is able to protect the heart from free radical-induced electrophysiological abnormalities and decreases the incidence of arrhythmias[72]. Heart attacks and strokes involve a disruption of the oxygen supply to the affected areas. When the oxygen supply is restored a burst of free radicals is produced which can cause additional tissue damage (ischemia-reperfusion injury). Animal experiments have shown that alpha-lipoic acid is effective in preventing or ameliorating ischemia-reperfusion injury[67]. The sulfur group in alpha-lipoic acid also makes it an excellent mercury detoxifier. It is very safe and I take 100 mg with breakfast and lunch. That's it for part I of the supplements for afibbers. In part II we will cover magnesium, l- carnitine, cayenne pepper and other more exotic supplements. Please stay tuned!
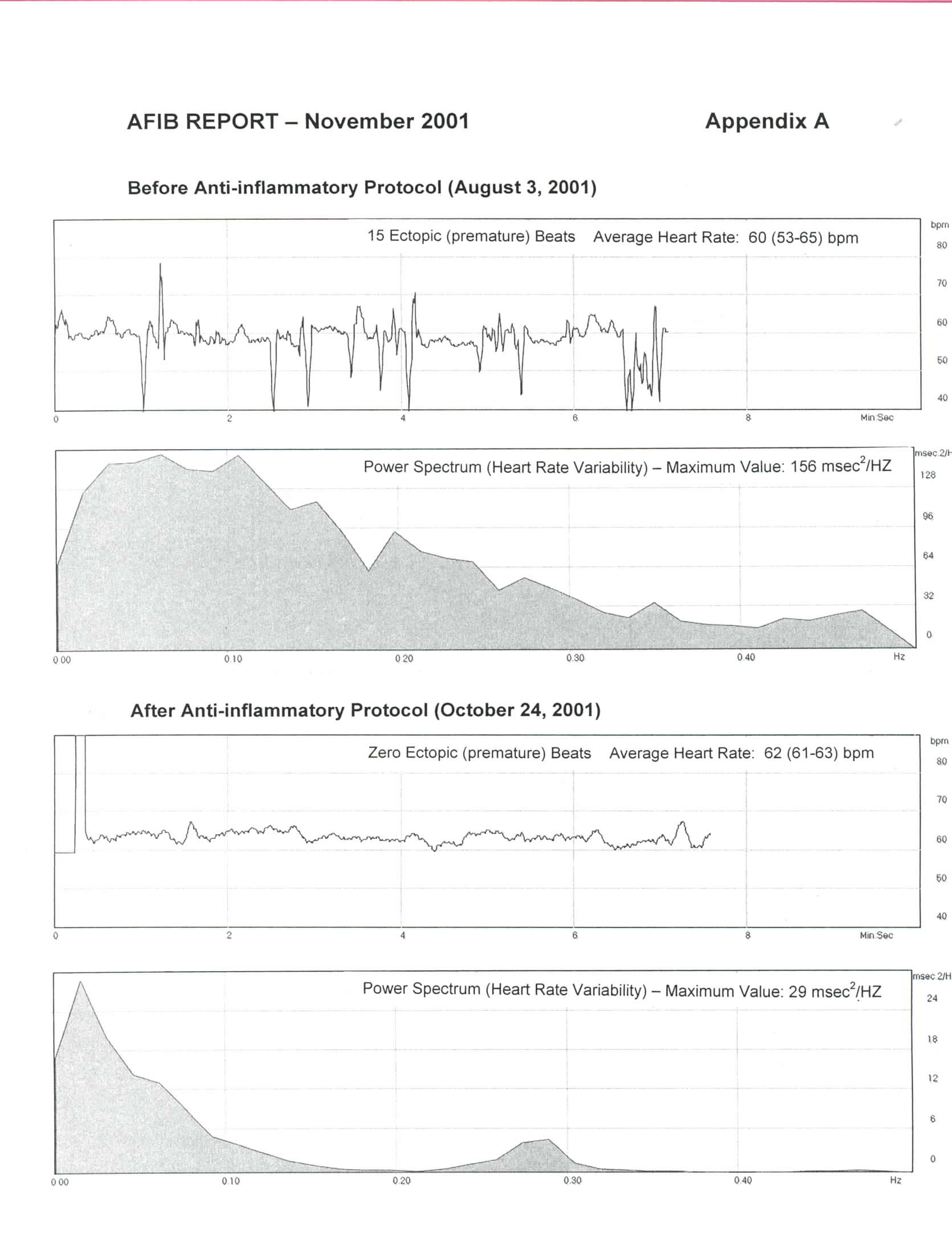
I began the anti-inflammation (Larsen) protocol described in the September 2001 issue of The AFIB Report on August 4th. I found it somewhat hard to completely eliminate all wheat and dairy products (except for butter), but eventually managed to do so once my wife found a great recipe for cookies made with quinoa flour! I now believe this was a very important first step. I also moderated my exercise program and increased my protein intake as per the blood type O diet. I had no problems taking the Moducare capsules on an empty stomach, but found the curcumin/bromelain combination to be quite irritating to the stomach so I switched to taking them with meals. I had originally included the herb Boswellia in my program, but found it gave me a headache so I discontinued it after 2 days. Throughout the protocol I measured my pulse rate, number of ectopic beats over a 5-minute period, heart rate variability, and autonomic nervous system balance daily using a fingertip pulse monitor and software program (FreezeFra mer) developed by the HeartMath Institute in California. I won't go into the details of the procedure at this time, but will cover it in detail in a future issue of The AFIB Report. Suffice it to say that on August 3rd I counted 15 ectopic beats (over 5 minutes) and my maximum power spectrum value was 156 milliseconds squared/Hertz – normal is about 8 to 30. So in other words, my heart's performance was rather chaotic. A month later on September 3rd things had changed quite remarkably. I recorded no ectopic beats and the maximum power spectrum value had decreased to 12. I also felt great, but the balance between the sympathetic (adrenergic) and parasympathetic branches was still a bit unusual. On September 19th I reduced my Moducare intake to one capsule three times a day instead of the two capsules three times a day I had been on since I began the protocol. Coincidence or not, I don't know, but early in the morning on September 21st I experienced my first LAF episode in 2 months. All veteran afibbers will know what a huge disappointment that was! I carried on with the protocol continuing to take three Moducare capsules a day, but reducing the curcumin/bromelain intake to just once a day (at lunch). It is now 5 weeks since my last episode and I am feeling extremely well. I am regularly clocking zero ectopic beats, my maximum power spectrum value is around 28 – within the normal range – and my autonomic nervous system balance is perfectly normal. As the saying goes, a picture is worth a thousand words, so to illustrate my progress I have included the tracings of my August 3rd test and those of today (October 24th) in the attached Appendix A. I am sure you will notice the difference. My plan now is to continue with the modified anti-inflammation program until I have gone 3 months without an episode and then re-evaluate the situation. Please, if you try the protocol, let me know how you make out.
References
|
The AFIB REPORT is published monthly by Hans R. Larsen MSc ChE 1320 Point Street Victoria, BC, Canada V8S 1A5 Phone: (250) 384-2524 E-mail: [email protected] URL: http://www.yourhealthbase.com Copyright © 2001 by Hans R. Larsen The AFIB REPORT does not provide medical advice. Do not attempt self- diagnosis or self-medication based on our reports. Please consult your health-care provider if you wish to follow up on the information presented. |